Consistent and cohesive messages extend brand awareness
One of the foundations of brand building is consistently delivering cohesive messages, in line with the brand purpose and values, to a variety of audience types. This begins with a sound partnership, working in tandem with our client to identify and deliver on opportunities to extend the brand and brand awareness to the audiences we most want to reach.
Our work with Intercept focuses primarily on recruiting and retaining subjects and the sites that treat them, in a series of NASH (Non-Alcoholic Steatohepatitis) clinical trials. Although NASH is a serious disease that can be life-threatening, it is a relative “baby” in terms of recognized diseases with high population impact. The current prevalence of NASH in the US is estimated be 2-12%¹ and the CDC estimates that by 2020 NASH will be the leading cause of liver transplant.²
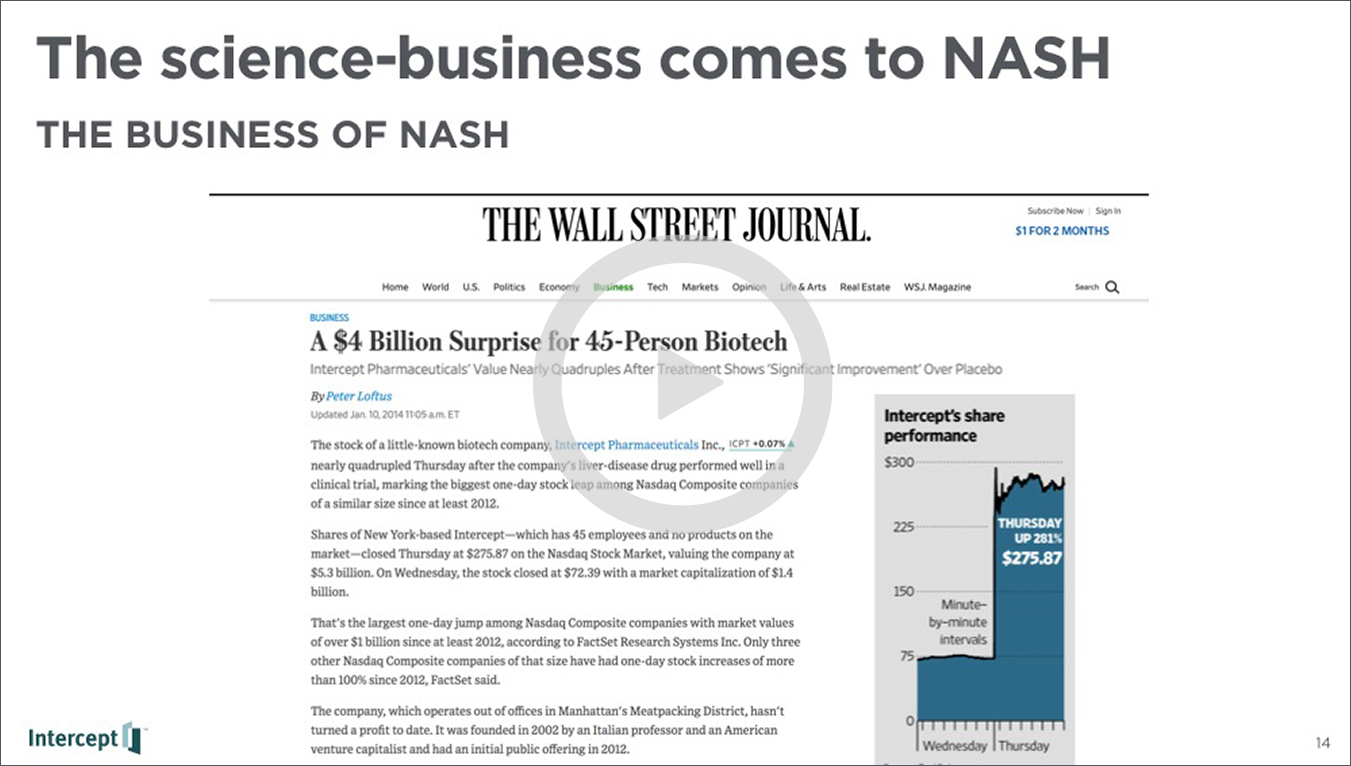
The ever-growing group of researchers, doctors, pharmaceutical and biotech companies, and healthcare advocates in search of a solution for NASH are blazing trails towards treatment options. Intercept has been on the front-lines of the effort from the start. In 2014 their product Obeticholic Acid (OCA), received breakthrough status by the FDA in a Phase II trial run by the National Institute of Diabetes & Digestive & Kidney Diseases (NIDDK). This designation was a real eye-opener to the market and the space became flooded with interest, NDA’s, and early trials. That was only 4 years ago.
In partnering with Intercept, we turned our practice of recruitment and retention communication to the audience of the NASH Summit, a conference of over 150 attendees from more than 120 organizations in its 2nd year of existence. This audience of scholars and researchers is highly inspired to find solutions or advances in resolving this disease. What speaks to this group is recognition of how new this disease is in terms of research and understanding, learning and working with cutting-edge science, and how quickly interest has grown and shaped the space with novel technologies and a new world of regulatory standards.
For the NASH summit, we partnered with the brilliant and articulate Jason Campagna, the Senior Vice President and Global NASH Lead at Intercept, who was the event keynote speaker, to build his presentation. His address guided the audience on a narrative journey supported by a dense visual timeline of all NASH clinical trials to date, highlighting milestones and developments, as well as MOAs and combinations that have sprung up along the way.
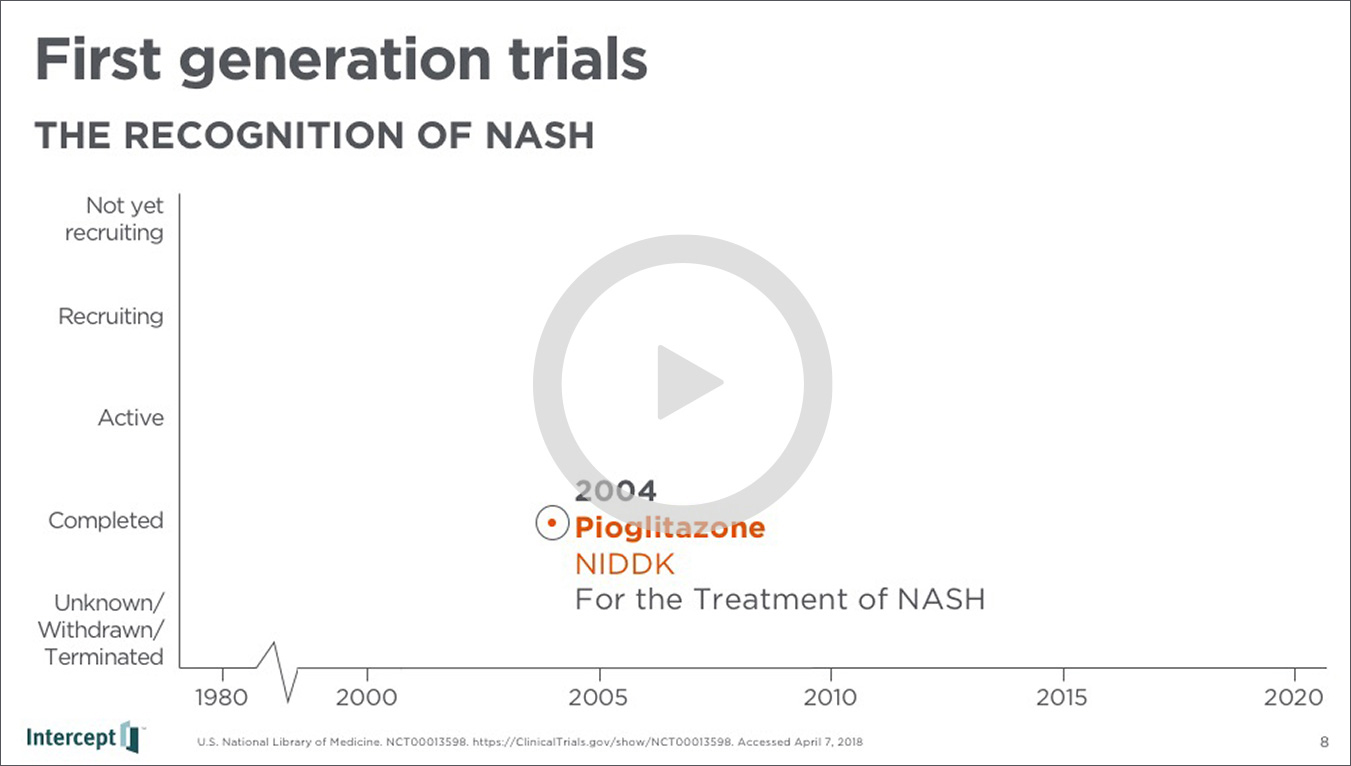
The presentation then looked to the business development in the space, attributing the flood in the market in part to OCA’s breakthrough designation, which turned attention from existing solutions (like diabetes drugs) to a flood of new science, new diagnostic technologies, and massive mergers and acquisitions.
The presentation ended with an appeal to the audience, that they not lose sight of the patient amidst the pomp of innovation. We emphasized the importance of understanding patients’ preferences, motivations, and situations in order to formulate solutions patients can actually manage. We urged them to design and develop real-world tools and processes that support and build empathy in clinical settings and invited them to see that creating positive patient experiences should be as much a focus as safety and efficacy.

It was through the cohesive messages created for the audience that reinforced Intercept’s purpose which is focused on developing and bringing to market, solutions for patients that suffer with non-viral liver diseases like NASH, that we were able to bolster their brand and extend positive brand awareness.
- Williams CD, Stengel J, Asike MI, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011 Jan;140(1):124-31.
- Wree A, Broderick L, Canbay A, et al. From NAFLD to NASH to cirrhosis-new insights into disease mechanisms. Nature reviews Gastroenterology & hepatology. 2013 Nov;10(11):627-36.
